Exploring the World's Most Explosive Chemicals
Written on
Chapter 1: The Most Explosive Chemical
At the pinnacle of explosive compounds is a highly energetic material known as 1-Diazidocarbamoyl-5-azidotetrazole, or C2N14, often referred to as “azidoazide azide.” This compound is remarkable for its structure, comprising 14 nitrogen atoms and two carbon atoms, making it extraordinarily reactive. In fact, C2N14 is so volatile that it can detonate in nearly any scenario, even without direct contact.
In a daring 2011 experiment, German scientists discovered that merely touching, moving, or dissolving this chemical could lead to an explosion. Even seemingly benign tests, like capturing its infrared spectrum, proved to be perilous.
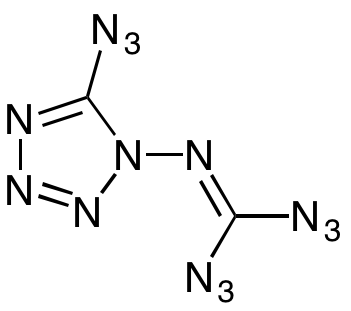
C2N14 exhibits strong endothermic properties, meaning it absorbs heat during reactions, and its chemical bonds are highly unstable. The 14 nitrogen bonds contribute significantly to its explosive nature, rendering it nearly impossible to measure safely. The only successful analysis conducted was an x-ray of its crystal arrangement. Due to its extreme volatility, only minuscule amounts have been synthesized, and it remains devoid of practical applications beyond its explosive potential. This is one chemical that researchers are unlikely to revisit.
Section 1.1: Other Hazardous Chemicals
Another chemical that competes with C2N14 in terms of danger is chlorine trifluoride. This substance is not only highly corrosive but also ignites nearly everything it encounters, including materials typically deemed non-flammable. Fires caused by chlorine trifluoride are notoriously difficult to extinguish.
Storage of this compound necessitates containers made from nickel, copper, iron, or steel, which must be treated with fluorine gas to form a protective layer that prevents oxidation. In a notable incident during the 1950s, a steel container of chlorine trifluoride was inadvertently cooled with dry ice, causing the container to become brittle. When the chemical leaked, it burned through a foot (approximately 30 centimeters) of concrete and three feet (about 90 centimeters) of gravel, producing corrosive fumes that damaged everything in proximity. In the 1990s, it found a niche in the semiconductor industry for cleaning tool chambers.
Subsection 1.1.1: Dimethyl Cadmium
Lastly, dimethyl cadmium is infamous not for its explosive capabilities but for its extreme toxicity. If this chemical is improperly stored, it can either ignite and release toxic cadmium oxide fumes or react with oxygen to create a hazardous dimethyl cadmium peroxide crust, which may explode. Even a few micrograms of dimethyl cadmium vapor pose severe health risks, potentially harming the liver, kidneys, heart, and lungs. Moreover, cadmium is recognized as a carcinogen.
The Strongest Acid in Existence
The title of the strongest acid belongs to a substance capable of dissolving nearly anything in mere moments, even organic material.
This video titled "The 5 Most Dangerous Chemicals on Earth" delves deeper into the perilous properties of various chemicals, including C2N14 and chlorine trifluoride.
Additionally, the video "The Most DESTRUCTIVE Chemical Reaction from Two NON-explosive Components" explores fascinating chemical reactions and their outcomes.