From Chemistry Cringe to Mendeleev's Marvel: A New Perspective
Written on
Understanding the Chemistry Mentalist
For many, the term “chemistry” can evoke a sense of dread, often reminding them of challenging undergraduate classes that were quickly forgotten after finals. However, this series aims to shift that perception, making the subject accessible and entertaining.
With the debate over atomic weights settled, chemists turned their attention to another pressing issue: the classification of elements. Antoine Lavoisier attempted this categorization in the late 1700s when only about 30 elements were recognized. As the list grew to approximately 60 by the 1860s, it sparked further attempts to organize them. Chemists were eager to uncover patterns in the elements' behaviors, as these patterns are crucial for making predictions—a fundamental aspect of chemistry.
In my previous discussion, I highlighted chemistry’s most notable number; now, it's time to delve into its most iconic table: the periodic table of elements. To properly address this, we must first acknowledge the genius behind it, Dmitri Mendeleev.

Mendeleev’s remarkable beard is unforgettable.
Once the scientific community agreed on atomic weights, the logical next step was to classify elements according to these weights. Various early periodic tables emerged, some with unusual designs—like a helical format—in an effort to group elements based on observed patterns. You might be wondering what these patterns are; hold that thought while we explore Mendeleev's contributions.
Hailing from Russia, Mendeleev was born to a schoolteacher and a glass factory worker, exemplifying how brilliance can arise from modest origins. After excelling in his studies, he earned a fellowship that took him to Germany for the Karlsruhe Congress, where he developed an obsession with the properties of elements. He meticulously crafted flashcards to categorize and rearrange the elements based on similarities, culminating in the publication of his first periodic table in 1869. This initial table was vertical but required further refinement.
Mendeleev successfully established groups based on chemical properties such as valency—the number of bonds an atom can form. For instance, carbon has a valency of four, as does silicon, leading him to group these elements together. He also identified periodicity, which referred to the gradual increase in atomic weight. For example, potassium (K, atomic weight 40), rubidium (Rb, atomic weight 85), and cesium (Cs, atomic weight 133) exhibit a pattern with about 45 atomic weight units separating them, justifying their classification together as they share similar valency.
Two years later, Mendeleev released a refined, horizontal version of the periodic table—one closely resembling the modern version. Grouping elements proved to be a complex task, complicated by inaccuracies in atomic weights. Nevertheless, Mendeleev showcased the value of his table in two significant ways: he corrected erroneous atomic weights and made predictions about undiscovered elements.
This latter achievement solidified Mendeleev’s legacy in the world of chemistry. In his 1871 periodic table, take note of the empty slot in row 4, group 3 (marked “— =44”), as well as similar placeholders in row 5, groups 3 and 4.
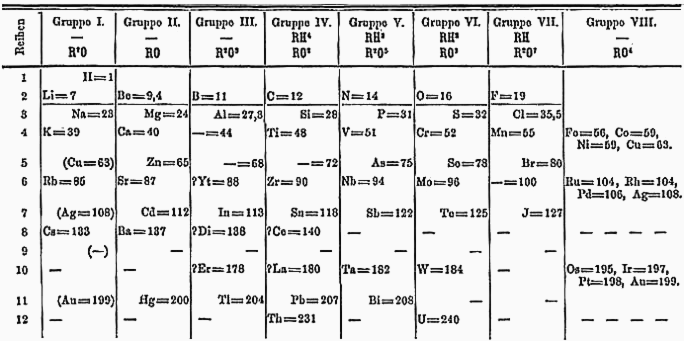
Mendeleev's famous 1871 periodic table | License
These vacant positions were assigned the prefix “eka” (meaning “first” in Sanskrit) in reference to their neighboring group members. He predicted the properties and atomic weights of eka-boron, eka-aluminum, and eka-silicon. The eventual discovery of gallium (eka-aluminum, 1875), scandium (eka-boron, 1879), and germanium (eka-silicon, 1886) with properties that perfectly matched his predictions piqued the interest of chemists worldwide.
Some even regarded him as a sort of psychic—almost like a mentalist in the realm of chemistry.
Works Cited:
Brock, William H. The Chemical Tree: A History of Chemistry. New York: Norton and Co, 2000.
Ihde, Aaron J. The Development of Modern Chemistry. New York: Dover Publications, 1984.
Chapter 2: The Legacy of Mendeleev
The first video explores the fascinating concept of the "Chemical Mentalist," delving into how Mendeleev's insights transformed the field of chemistry.
The second video presents the track "Chemical Mentalist" from Fast X (2023), showcasing a vibrant soundtrack that complements the exploration of Mendeleev's work.